ahc
Premier Ampoule Eye Cream For Face Line Tightening

Product overview
Brand: AHC
Product Name: Premier Ampoule Eye Cream For Face Line Tightening (also referred to as “Season 13”)
Korean Product Name: 프리미어 앰플 아이크림 포 페이스 라인 타이트닝
Capacity: 40ml
Formulated by: Kolmar Korea
Manufactured by: Kolmar Korea
Distributed by: Unilever Carver Korea
Country of Manufacture: South Korea
Functional Cosmetic Certifications: Anti-Wrinkle and Whitening Dual Functional Cosmetic
Original Retail Price: 33,000 KRW
Date of Release: January 2025
Shelf life: 3 years before opening. 12 months after opening.
Ingredients
Notable allergens: Fragrance, Niacinamide.
Product description
Experience unparalleled elasticity with threads designed to defy gravity: AHC’s NEW “Premier Ampoule Eye Cream For Face Line Tightening”1.
[1] The expression “Line Tightening” refers to the reduction of the appearance of wrinkle lines.
Number 1 eye cream
AHC’s “Eye Cream For Face” has been the number 1 eye cream for eight consecutive years2, with total sales surpassing 130 million units.
The renewed 13th edition of AHC’s “Eye Cream For Face”, powered by Bonding Collagen T7.
[2] This ranking is based on data from Kantar Worldpanel, which indicates that AHC has maintained its No.1 position from 20 June 2016 to 16 June 2024, based on total eye cream purchase volume.
Full-face skin tightening
AHC’s “Premier Ampoule Eye Cream For Face Line Tightening” offers a comprehensive skin tightening treatment designed to effectively firm and improve the elasticity of the entire face.
With the passage of time, skin elasticity gradually declines, leading to the emergence of new skin concerns.
SOS Skin Elasticity Checklist:
If two or more of these points resonate with you, it’s time to consider a more powerful, targeted anti-aging treatment!
Boost your skin’s elasticity with a single cream
AHC’s “Premier Ampoule Eye Cream For Face Line Tightening”: formulated with Bonding Collagen T7 and Peptide threads.
[3] The expression ‘Within the skin” refers to the epidermal layer.
[4] The expression “Line Tightening” refers to the reduction of the appearance of wrinkle lines.
Comprehensive skin-firming solution
AHC’s “Premier Ampoule Eye Cream For Face Line Tightening” simultaneously enhances the elasticity of eight different areas of the skin across the entire face5.
A powerful skin-firming cream supported by in-vivo test results:
[5] Clinical test to evaluate the enhancement of skin elasticity in eight specific sagging areas: forehead, glabella, periocular area, infraorbital area (eye bags), eyelids, cheeks, perioral area, and neck.
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 33 women aged 40-60.
Date: 28 August – 27 September 2024.
Treatment areas: forehead, glabella, periocular area, infraorbital area (eye bags), eyelids, cheeks, perioral area, and neck.
Application protocol: Participants applied the test product to the designated treatment areas for four weeks.
Individual results may vary.
AHC’s proprietary line tightening technology6
Discover the powerful effects of AHC’s “Premier Ampoule Eye Cream For Face Line Tightening”.
face wrinkles:
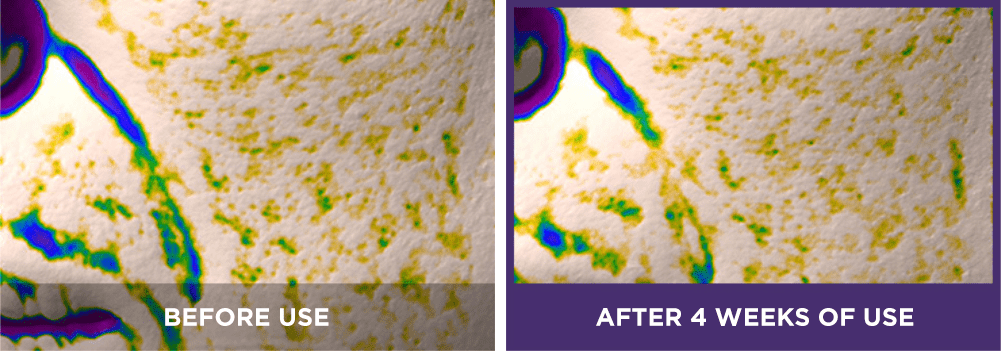
Perioral area:
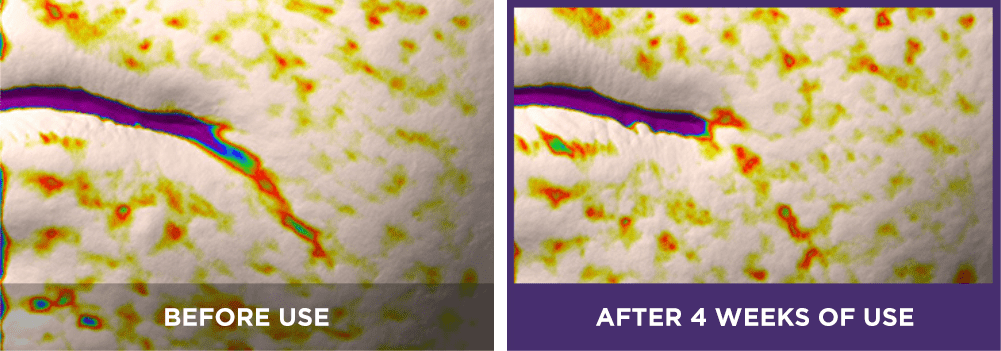
Double chin:
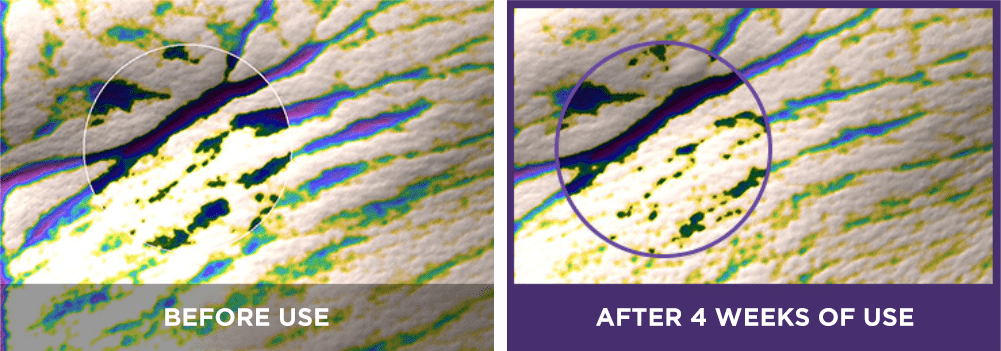
skin elastic resilience:
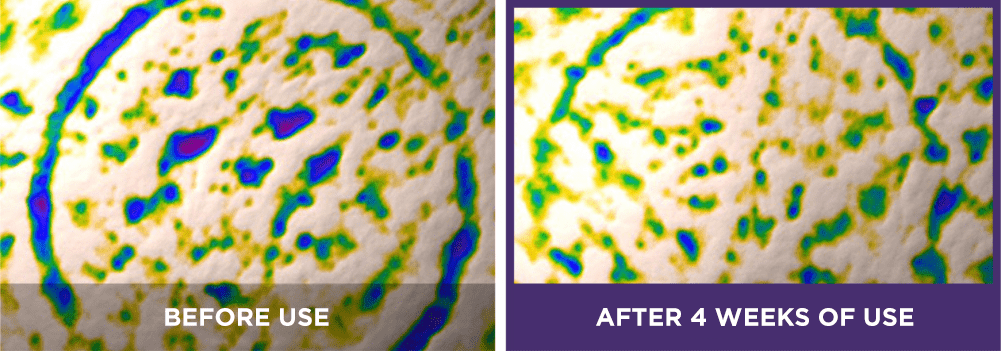
[6] The expression “Line Tightening” refers to the reduction of the appearance of wrinkle lines.
Proven
AHC’s “Premier Ampoule Eye Cream For Face Line Tightening” offers a skin-firming and lifting effect so impressive that others will notice it before you do.
woman in her 40s:

Full-face skin tightening
AHC’s “Premier Ampoule Eye Cream For Face Line Tightening” is a full-face skin-tightening treatment designed to effectively firm your skin.
Three-step formula:
[7] Oligopeptide-6.
Step 1: Penetrate
Through the use of Nano-Hyasome technology, AHC’s “Premier Ampoule Eye Cream For Face Line Tightening” features an ultra-fine moisturising formula that is 1/1300 smaller than skin pores.
Point 1: AHC’s “Premier Ampoule Eye Cream For Face Line Tightening” boasts a formula that is 1/1300 smaller than skin pores8, making it the brand’s finest formula to date8. This ultra-fine formula penetrates the skin 167% deeper and 167% faster9.

[8] Pore size may vary from person to person. This comparison is based on the formula sizes of AHC’s “Eye Cream For Face” Seasons 4–12.
[9] This measurement refers solely to the epidermal layer and is in comparison to AHC’s “Premier Ampoule In Eye Cream Core Lifting”.
Clinical test to evaluate the enhancement of skin permeation, permeation depth, and permeation kinetics.
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 30 adults aged 20-55.
Date: 27 August – 10 October 2024.
Treatment areas: facial region.
Application protocol: Participants applied the test and control products to the designated treatment areas, with skin absorption assessed immediately and 30 minutes after application.
Control product: AHC’s “Premier Ampoule In Eye Cream Core Lifting” served as the benchmark for comparison.
Individual results may vary.
Point 2: The particles of the formula are wrapped twice in hyaluronic acid to provide a rapid moisturising effect similar to the benefits of using a sheet mask every day.

Step 2: Replenish
AHC’s “Premier Ampoule Eye Cream For Face Line Tightening” is formulated with Bonding Collagen T7, a potent ingredient discovered by AHC, a specialist in anti-aging solutions.
Introducing the NEW AHC’s “Premier Ampoule Eye Cream For Face Line Tightening” with twice10 the collagen content of the previous season.
From the AHC Laboratory: Witness the firming effects of Bonding Collagen T7 for yourself.

[10] The product contains twice the collagen content of AHC’s “Premier Ampoule In Eye Cream Core Lifting”.
[11] In comparison to six human-like collagens (T1, T13, T4, T17, T21), Bonding Collagen T7 has active-site amino acid sequences that align with those found in human collagen genes, specifically COL1A2, COL3A1, COL-IA1, COL5A1, COL17A1, and COL21A1.
[12] This expression refers to the most pronounced wrinkles found in a specific area or region of the face.
[13] Clinical test to evaluate the enhancement of skin elasticity and the maintenance of this improvement 36 hours after discontinuation of product use.
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 30 adults aged 20-55.
Date: 27 August – 10 October 2024.
Treatment areas: cheek area.
Application protocol: Participants applied the test and control products to the designated treatment areas, with skin elasticity assessed immediately, two weeks after use, four weeks after use and 36 hours after discontinuing use.
Control product: AHC’s “Premier Ampoule In Eye Cream Core Lifting” served as the benchmark for comparison.
Individual results may vary.
Step 3: Tighten
Peptide threads designed to tighten skin in one go. AHC’s “Premier Ampoule Eye Cream For Face Line Tightening” is formulated with freeze-dried peptide threads.
[14] In comparison to AHC’s “Premier Ampoule In Eye Cream Core Lifting”.
Clinical test to evaluate the product’s effectiveness in improving skin sagging (elasticity lifting) in the mandibular area at twice the speed of the control product.
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 30 adults aged 20-55.
Date: 27 August – 10 October 2024.
Treatment areas: lateral facial region positioned at a 45-degree angle from the center of the face.
Application protocol: Participants applied the test and control products to the designated treatment areas, with skin lifting assessed two weeks after use and four weeks after use.
Control product: AHC’s “Premier Ampoule In Eye Cream Core Lifting” served as the benchmark for comparison.
Individual results may vary.
[15] In comparison to a blank control.
Clinical test to evaluate the enhancement of skin elastic resilience (fold increase).
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 32 women aged 40-60.
Date: 4 September – 7 October 2024.
Treatment areas: cheek area.
Application protocol: Participants applied the test product to the designated treatment areas for four weeks.
Individual results may vary.
Lightweight, non-sticky formula
AHC’s “Premier Ampoule Eye Cream For Face Line Tightening” features a lightweight, non-sticky formula that rapidly absorbs into the skin, providing deep hydration. A single eye cream contains ten bottles of AHC’s “Premier Collagen T7 Ampoule”16!
[16] AHC’s “Premier Collagen T7 Ampoule” is an ampoule that contains the same collagen ingredient found in the eye cream.
Texture
AHC’s “Premier Ampoule Eye Cream For Face Line Tightening” glides smoothly over the skin, allowing its highly concentrated formula to seamlessly blend into the face, leaving it firmer and more elastic.
3D massage tip applicator
A 3D massage tip applicator designed to help you achieve firmer skin with ease.
AHC’s “Premier Ampoule Eye Cream For Face Line Tightening” features a sculpt-tip applicator that allows you to address dullness, wrinkles, and firmness all at once.
Tip 1: Vitality — Dot Massage

Use the applicator to apply firm pressure on the facial acupressure points.
Tip 2: Wrinkles — Line Massage

Gently trace lines over the forehead, between the eyebrows, the outer corners of the eyes, the nasolabial folds, and the philtrum to relax each area.
Tip 3: Elasticity — Surface Massage

Massage from the corners of the mouth to the ears in an upward motion, as if you are lifting the skin of the entire face.
How to use
Full face skin tightening routine. AHC’s “Premier Ampoule Eye Cream For Face Line Tightening” is an eye cream designed to enhance the elasticity of the entire face in one go.
Clinical tests
Full face skin tightening with a single eye cream:
1. Clinical test to evaluate the product’s effectiveness in improving skin sagging (elasticity lifting) in the mandibular area at twice the speed of the control product.
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 30 adults aged 20-55.
Date: 27 August – 10 October 2024.
Treatment areas: lateral facial region positioned at a 45-degree angle from the center of the face
Application protocol: Participants applied the test and control products to the designated treatment areas, with skin lifting assessed two weeks after use and four weeks after use.
Control product: AHC’s “Premier Ampoule In Eye Cream Core Lifting” served as the benchmark for comparison.
Individual results may vary.
2. Clinical test to evaluate the enhancement of skin lifting in sagging cheek area.
Tests conducted by: MarieDM Skin Research Center
Participants: 30 women aged 44-59.
Date: 2 September – 4 October 2024.
Treatment areas: facial region.
Application protocol: Participants applied the test product to the designated treatment areas for four weeks.
Individual results may vary.
3. Clinical test to evaluate the enhancement of skin elasticity in eight specific sagging areas: forehead, glabella, periocular area, infraorbital area (eye bags), eyelids, cheeks, perioral area, and neck.
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 33 women aged 40-60.
Date: 28 August – 27 September 2024
Treatment areas: forehead, glabella, periocular area, infraorbital area (eye bags), eyelids, cheeks, perioral area, and neck.
Application protocol: Participants applied the test product to the designated treatment areas for four weeks.
Individual results may vary.
4. Clinical test to evaluate the enhancement of skin elasticity lifting in seven specific sagging areas: forehead, periocular area, cheeks, perioral area, mandibular area, glabella, infraorbital area.
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 33 women aged 40-60.
Date: 28 August – 27 September 2024
Treatment areas: forehead, periocular area, cheeks, perioral area, mandibular area, glabella, infraorbital area.
Application protocol: Participants applied the test product to the designated treatment areas, with skin lifting assessed two weeks after use and four weeks after use.
Individual results may vary.
5. Clinical test to evaluate the enhancement of skin sagging (elasticity lifting).
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 31 women aged 40-59.
Date: 27 August – 8 October 2024
Treatment areas: facial region (perioral area).
Application protocol: Participants applied the test product to the designated treatment areas, with skin lifting assessed two weeks after use and four weeks after use.
Individual results may vary.
Full face wrinkle treatment with a single eye cream:
6. Clinical test to evaluate the immediate (temporary) improvement in elasticity and tightening of sagging (enlarged) pore areas, encompassing both horizontal and vertical pores.
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 33 women aged 40-60.
Date: 28 August – 27 September 2024
Treatment areas: cheek area.
Application protocol: Participants applied the test product to the designated treatment areas, with skin tightening assessed immediately after use, after two weeks of use and after four weeks of use.
Individual results may vary.
7. Clinical test to evaluate the enhancement of skin elastic resilience (percentage increase).
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 32 women aged 40-60.
Date: 4 September – 7 October 2024.
Treatment areas: cheek area.
Application protocol: Participants applied the test product to the designated treatment areas, with elastic skin resilience assessed immediately after use, after two weeks of use and after four weeks of use.
Individual results may vary.
8. Clinical test to evaluate the enhancement of skin elastic resilience (fold increase).
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 32 women aged 40-60.
Date: 4 September – 7 October 2024.
Treatment areas: cheek area.
Application protocol: Participants applied the test product to the designated treatment areas for four weeks.
Individual results may vary.
9. Clinical test to evaluate the skin turgor response time.
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 32 women aged 40-60.
Date: 4 September – 7 October 2024.
Treatment areas: cheek area.
Application protocol: Participants applied the test product to the designated treatment areas, with skin turgor assessed immediately after use, after two weeks of use and after four weeks of use.
Individual results may vary.
10. Clinical test to evaluate the reduction of the appearance of deep wrinkles that have developed over a span of four years.
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 30 adults aged 20-55. All participants have previously participated in eye wrinkle clinical studies at least four years prior to this evaluation.
Date: 27 August – 10 October 2024.
Treatment areas: periocular area.
Application protocol: Participants applied the test product to the designated treatment areas for four weeks
Individual results may vary.
11. Clinical test to evaluate the reduction of the appearance of skin wrinkles.
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 31 women aged 40-59.
Date: 27 August – 8 October 2024.
Treatment areas: facial region (perioral area).
Application protocol: Participants applied the test product to the designated treatment areas, with wrinkles assessed two weeks after use and four weeks after use.
Individual results may vary.
12. Clinical test to evaluate the reduction of sagging wrinkles across the entire face.
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 33 women aged 40-60.
Date: 28 August – 27 September 2024.
Treatment areas: entire facial region.
Application protocol: Participants applied the test product to the designated treatment areas, with sagging wrinkles assessed after two weeks of use and after four weeks of use.
Individual results may vary.
13. Clinical test to evaluate the reduction of sagging deep wrinkles across eight specific areas(forehead, glabella, lateral canthus area, infraorbital area, eyelids, philtrum, perioral area, neck).
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 33 women aged 40-60.
Date: 28 August – 27 September 2024.
Treatment areas: forehead, glabella, lateral canthus area, infraorbital area, eyelids, philtrum, perioral area, neck.
Application protocol: Participants applied the test product to the designated treatment areas for four weeks.
Individual results may vary.
14. Clinical test to evaluate the reduction of deep wrinkle areas across seven specific areas (forehead, glabella, lateral canthus area, infraorbital area, philtrum, perioral area, neck).
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 33 women aged 40-60.
Date: 28 August – 27 September 2024.
Treatment areas: forehead, glabella, lateral canthus area, infraorbital area, philtrum, perioral area, neck.
Application protocol: Participants applied the test product to the designated treatment areas for four weeks.
Individual results may vary.
15. Clinical test to evaluate the reduction in the number of deep sagging wrinkles across seven specific areas (forehead, glabella, lateral canthus area, infraorbital area, philtrum, perioral area, neck).
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 33 women aged 40-60.
Date: 28 August – 27 September 2024.
Treatment areas: forehead, glabella, lateral canthus area, infraorbital area, philtrum, perioral area, neck.
Application protocol: Participants applied the test product to the designated treatment areas for four weeks.
Individual results may vary.
16. Clinical test to evaluate the enhancement of skin brightness in wrinkle areas.
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 33 women aged 40-60.
Date: 28 August – 27 September 2024.
Treatment areas: periocular area.
Application protocol: Participants applied the test product to the designated treatment areas, with skin brightness assessed before use, immediately after use, two weeks after use, and four weeks after use.
Individual results may vary.
Twice the anti-aging benefits compared to the previous season, including a decrease in aging acceleration and a lowered skin aging index:
17. Clinical test to evaluate the reduction of dynamic wrinkles in the perioral area.
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 32 women aged 40-60.
Date: 4 September – 7 October 2024.
Treatment areas: perioral area.
Application protocol: Participants applied the test and control products to the designated treatment areas, with dynamic wrinkles assessed two weeks after use and four weeks after use.
Control product: AHC’s “Premier Ampoule In Eye Cream Core Lifting” served as the benchmark for comparison.
Individual results may vary.
18. Clinical test to evaluate the reduction of crow’s feet wrinkles and the maintenance of this improvement 36 hours after discontinuation of product use.
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 30 adults aged 20-55.
Date: 27 August – 10 October 2024.
Treatment areas: periocular area.
Application protocol: Participants applied the test and control products to the designated treatment areas, with crow’s feet wrinkles assessed two weeks after use, four weeks after use and 36 hours after discontinuing use.
Control product: AHC’s “Premier Ampoule In Eye Cream Core Lifting” served as the benchmark for comparison.
Individual results may vary.
19. Clinical test to evaluate the enhancement of skin moisture and the maintenance of this improvement 36 hours after discontinuation of product use.
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 30 adults aged 20-55.
Date: 27 August – 10 October 2024.
Treatment areas: cheek area.
Application protocol: Participants applied the test and control products to the designated treatment areas, with skin elasticity assessed immediately, two weeks after use, four weeks after use and 36 hours after discontinuing use.
Control product: AHC’s “Premier Ampoule In Eye Cream Core Lifting” served as the benchmark for comparison.
Individual results may vary.
20. Clinical test to evaluate the enhancement of skin elasticity and the maintenance of this improvement 36 hours after discontinuation of product use.
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 30 adults aged 20-55.
Date: 27 August – 10 October 2024.
Treatment areas: cheek area.
Application protocol: Participants applied the test and control products to the designated treatment areas, with skin elasticity assessed immediately after use, two weeks after use, four weeks after use and 36 hours after discontinuing use.
Control product: AHC’s “Premier Ampoule In Eye Cream Core Lifting” served as the benchmark for comparison.
Individual results may vary.
21. Clinical test to evaluate the enhancement of skin lifting of the facial region (cheek area) and the maintenance of this improvement 36 hours after discontinuation of product use.
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 30 adults aged 20-55.
Date: 27 August – 10 October 2024.
Treatment areas: cheek area.
Application protocol: Participants applied the test and control products to the designated treatment areas, with skin lifting assessed four weeks after use and 36 hours after discontinuing use.
Control product: AHC’s “Premier Ampoule In Eye Cream Core Lifting” served as the benchmark for comparison.
Individual results may vary.
22. Clinical test to evaluate the enhancement of skin density and the maintenance of this improvement 36 hours after discontinuation of product use.
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 30 adults aged 20-55.
Date: 27 August – 10 October 2024.
Treatment areas: periocular area.
Application protocol: Participants applied the test and control products to the designated treatment areas, with skin density assessed five hours after use, four weeks after use and 36 hours after discontinuing use.
Control product: AHC’s “Premier Ampoule In Eye Cream Core Lifting” served as the benchmark for comparison.
Individual results may vary.
23. Clinical test to evaluate the reduction of skin aging index.
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 30 adults aged 20-55.
Date: 27 August – 10 October 2024.
Evaluation criteria: crow’s feet wrinkles, skin elasticity and skin texture.
Application protocol: Participants applied the test product for four weeks.
Individual results may vary.
Addresses multiple signs of aging at once:
24. Clinical test to evaluate the reduction of wrinkles on the dorsum of the hands.
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 31 women aged 40-59.
Date: 27 August – 8 October 2024.
Treatment areas: dorsal hands.
Application protocol: Participants applied the test product to the designated treatment areas, with wrinkles assessed two weeks after use and four weeks after use.
Individual results may vary.
25. Clinical test to evaluate the improvement of skin elasticity in mitigating immediate (transient) indentation marks caused by pillows, sunglasses, and hats.
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 31 women aged 40-59.
Date: 27 August – 8 October 2024.
Treatment areas: facial region (cheeks, nasal bridge, forehead).
Application protocol: Participants applied the test product to the designated treatment areas, with skin elasticity assessed before use (blank control)and immediately after use.
Individual results may vary.
An all-in-one skincare solution that delivers results using only half the quantity!
26. Clinical test to evaluate the effects of using half the amount of product compared to the full application of a control product (a 5-step skincare routine). The study assessed improvements in skin sagging, including enhanced elasticity and lifting effects.
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 31 women aged 40-59.
Date: 27 August – 8 October 2024.
Treatment areas: facial region (perioral area).
Application protocol: Participants applied the test product to the designated treatment areas, with skin sagging assessed two weeks after use and four weeks after use.
Control product: a 5-step skincare routine.
Individual results may vary.
27. Clinical test to evaluate the effects of using half the amount of product compared to the full application of a control product (a 5-step skincare routine). The study assessed reduction in the appearance of wrinkles.
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 31 women aged 40-59.
Date: 27 August – 8 October 2024.
Treatment areas: facial region (perioral area).
Application protocol: Participants applied the test product to the designated treatment areas, with skin sagging assessed two weeks after use and four weeks after use.
Control product: a 5-step skincare routine.
Individual results may vary.
Faster and deeper absorption:
28. Clinical test to evaluate the enhancement of skin permeation, permeation depth, and permeation kinetics.
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 30 adults aged 20-55.
Date: 27 August – 10 October 2024.
Treatment areas: facial region.
Application protocol: Participants applied the test and control products to the designated treatment areas, with skin absorption assessed immediately and 30 minutes after application.
Control product: AHC’s “Premier Ampoule In Eye Cream Core Lifting” served as the benchmark for comparison.
Individual results may vary.
Skin texture and density:
29. Clinical test to evaluate the enhancement of skin density (compactness).
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 31 women aged 40-59.
Date: 27 August – 8 October 2024.
Treatment areas: periocular area.
Application protocol: Participants applied the test product to the designated treatment areas, with skin density assessed one hour after use and four weeks after use.
Individual results may vary.
30. Clinical test to evaluate the improvement of wrinkle texture in pore areas.
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 30 adults aged 20-55. All participants have previously participated in eye wrinkle clinical studies at least four years prior to this evaluation.
Date: 27 August – 10 October 2024.
Treatment areas: Cheek area.
Application protocol: Participants applied the test product to the designated treatment areas, with wrinkle texture assessed immediately after use, two weeks after use and four weeks after use.
Individual results may vary.
31. Clinical test to evaluate the immediate (temporary) improvement of rough skin texture.
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 31 women aged 40-59.
Date: 27 August – 8 October 2024.
Treatment areas: cheek area.
Application protocol: Participants applied the test product to the designated treatment areas, with skin texture assessed immediately after use.
Individual results may vary.
32. Clinical test to evaluate the enhancement of skin texture.
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 30 adults aged 20-55. All participants have previously participated in eye wrinkle clinical studies at least four years prior to this evaluation.
Date: 27 August – 10 October 2024.
Treatment areas: Cheek area.
Application protocol: Participants applied the test product to the designated treatment areas, with wrinkle texture assessed immediately after use, two weeks after use and four weeks after use.
Individual results may vary.
Eye cream with additional moisturising benefits:
33. Clinical test to evaluate the immediate (temporary) improvement of skin moisture levels.
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 31 women aged 40-59.
Date: 27 August – 8 October 2024.
Treatment areas: cheek area.
Application protocol: Participants applied the test product to the designated treatment areas, with skin texture assessed immediately after use.
Individual results may vary.
34. Clinical test to evaluate the improvement in 48-hour moisture retention.
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 31 women aged 40-59.
Date: 27 August – 8 October 2024.
Treatment areas: forearm area.
Application protocol: Participants applied the test product to the designated treatment areas, with skin moisture retention assessed immediately after use and 48 hours after use.
Individual results may vary.
35. Clinical test to evaluate the enhancement of skin moisture levels comparable to using one sheet mask every day.
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 33 women aged 40-60.
Date: 28 August – 27 September 2024.
Treatment areas: neck area.
Application protocol: Participants applied the test product to the designated treatment areas for one week.
Individual results may vary.
Designed to address multiple concerns at once, including skin whitening [t/n skin brightening], dark spots, melasma, uneven skin tone, and lacklustre skin:
36. Clinical test to evaluate the efficacy in reducing the appearance and extent of areas affected by melasma and dark spots.
Tests conducted by: Korea Institute of Dermatological Sciences.
Participants: 32 women aged 28-66.
Date: 29 August – 21 October 2024.
Treatment areas: cheek area.
Application protocol: Participants applied the test product to the designated treatment areas, with skin moisture retention assessed immediately after use, three days after use, two weeks after use and four weeks after use.
Individual results may vary.
37. Clinical test to evaluate the reduction in the number of dark spots and areas affected by melasma.
Tests conducted by: Korea Institute of Dermatological Sciences.
Participants: 32 women aged 28-66.
Date: 29 August – 21 October 2024.
Treatment areas: cheek area.
Application protocol: Participants applied the test product to the designated treatment areas, with skin moisture retention assessed three days after use and four weeks after use.
Individual results may vary.
38. Clinical test to evaluate the reduction of the appearance of skin pigmentation, dark spots, and areas affected by melasma.
Tests conducted by: Korea Institute of Dermatological Sciences.
Participants: 32 women aged 28-66.
Date: 29 August – 21 October 2024.
Treatment areas: facial area.
Application protocol: Participants applied the test product to the designated treatment areas for four weeks.
Individual results may vary.
39. Clinical test to evaluate the enhancement of skin clarity.
Tests conducted by: Korea Institute of Dermatological Sciences.
Participants: 32 women aged 28-66.
Date: 29 August – 21 October 2024.
Treatment areas: facial area.
Application protocol: Participants applied the test product to the designated treatment areas, with skin clarity assessed immediately after use.
Individual results may vary.
40. Clinical test to evaluate the reduction of dark circles.
Tests conducted by: Korea Institute of Dermatological Sciences.
Participants: 32 women aged 28-66.
Date: 29 August – 21 October 2024.
Treatment areas: infraorbital area.
Application protocol: Participants applied the test product to the designated treatment areas for four weeks.
Individual results may vary.
41. Clinical test to evaluate the immediate (temporary) improvement of skin tonality across the overall complexion (encompassing forehead, periocular areas, and cheeks) in terms of brightness, yellow undertones, and dark undertones.
Tests conducted by: Korea Institute of Dermatological Sciences.
Participants: 32 women aged 28-66.
Date: 29 August – 21 October 2024.
Treatment areas: facial region.
Application protocol: Participants applied the test product to the designated treatment areas, with skin tonality assessed immediately after use.
Individual results may vary.
42. Clinical test to evaluate the immediate (temporary) improvement of skin radiance across the overall complexion (encompassing forehead, periocular areas, and cheeks).
Tests conducted by: Korea Institute of Dermatological Sciences.
Participants: 32 women aged 28-66.
Date: 29 August – 21 October 2024.
Treatment areas: facial region.
Application protocol: Participants applied the test product to the designated treatment areas, with skin radiance assessed immediately after use.
Individual results may vary.
Skin soothing and cooling:
43. Clinical test to evaluate the product’s effectiveness in inducing an immediate (temporary) decrease in skin temperature (cooling effect).
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 31 women aged 40-59.
Date: 27 August – 8 October 2024
Treatment areas: facial region.
Application protocol: Participants applied the test product to the designated treatment areas, with skin temperature assessed before and after infrared (heat) exposure, as well as immediately after product application.
Individual results may vary.
44. Clinical test to evaluate the product’s effectiveness in providing an immediate (temporary) skin soothing effect on skin irritation caused by infrared heat exposure.
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 31 women aged 40-59.
Date: 27 August – 8 October 2024
Treatment areas: facial region.
Application protocol: Participants applied the test product to the designated treatment areas, with skin temperature assessed before and after infrared (heat) exposure, as well as immediately after product application.
Individual results may vary.
Effects of using the product in conjunction with the massage tip applicator:
45. Clinical test to evaluate the immediate (temporary) reduction of surface melanin content in the infraorbital dark circle area when using the eye cream in conjunction with the applicator on the periocular area.
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 33 women aged 40-60.
Date: 28 August – 27 September 2024.
Treatment areas: infraorbital area.
Application protocol: Participants applied the test product to the designated treatment areas, with melanin content assessed after 30 minutes of use.
Individual results may vary.
46. Clinical test to evaluate the product’s effectiveness in providing an immediate (temporary) increase in blood circulation when using the eye cream in conjunction with the applicator.
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 33 women aged 40-60.
Date: 28 August – 27 September 2024.
Treatment areas: cheek area.
Application protocol: Participants applied the test product to the designated treatment areas, with blood circulation assessed immediately after use.
Individual results may vary.
47. Clinical test to evaluate the product’s effectiveness in delivering an immediate (temporary) improvement in the brightness and clarity of the periocular area when using the eye cream in conjunction with the applicator.
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 33 women aged 40-60.
Date: 28 August – 27 September 2024.
Treatment areas: periocular area.
Application protocol: Participants applied the test product to the designated treatment areas, with skin brightness and clarity assessed immediately after use.
Individual results may vary.
48. Clinical test to evaluate the product’s effectiveness in delivering an immediate (temporary) improvement in skin brightness when using the eye cream in conjunction with the applicator.
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 33 women aged 40-60.
Date: 28 August – 27 September 2024.
Treatment areas: cheek area.
Application protocol: Participants applied the test product to the designated treatment areas, with skin brightness assessed immediately after use.
Individual results may vary.
49. Clinical test to evaluate the immediate (temporary) reduction of deep wrinkles across seven specific areas (forehead, glabella, lateral canthus area, perioral area, philtrum, oral commissures, neck) when using the eye cream in conjunction with the applicator.
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 33 women aged 40-60.
Date: 28 August – 27 September 2024.
Treatment areas: forehead, glabella, lateral canthus area, perioral area, philtrum, oral commissures, neck.
Application protocol: Participants applied the test product to the designated treatment areas, with wrinkles assessed immediately after use.
Individual results may vary.
50. Clinical test to evaluate the immediate (temporary) enhancement of skin elasticity across the overall complexion (encompassing forehead, periocular areas, cheeks, perioral areas and mandibular area) when using the eye cream in conjunction with the applicator.
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 33 women aged 40-60.
Date: 28 August – 27 September 2024.
Treatment areas: forehead, periocular areas, cheeks, perioral areas and mandibular area.
Application protocol: Participants applied the test product to the designated treatment areas, with skin elasticity assessed immediately after use.
Individual results may vary.
51. Clinical test to evaluate the immediate (temporary) enhancement of skin sagging (measured by changes in angles) in the cheek area when using the eye cream in conjunction with the applicator.
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 33 women aged 40-60.
Date: 28 August – 27 September 2024.
Treatment areas: cheek area.
Application protocol: Participants applied the test product to the designated treatment areas, with skin sagging assessed immediately after use.
Individual results may vary.
In vitro test results:
52. In vitro assessment of the eye cream’s effectiveness in promoting collagen synthesis.
Tests conducted by: P&K Skin Research Center Co. Ltd.
Date: 2 September – 10 October 2024.
Test product: AHC’s “Premier Ampoule Eye Cream For Face Line Tightening” tested at concentrations of 0.0001%, 0.0005%, and 0.0010%, with differences observed by concentration.
53. In vitro assessment of the promotion of collagen synthesis over a 48-hour period.
Tests conducted by: P&K Skin Research Center Co. Ltd.
Date: 2-26 September 2024.
Test product: AHC’s “Premier Ampoule Eye Cream For Face Line Tightening” tested at concentrations of 0.0001%, 0.0005%, and 0.0010%, with differences observed by concentration at 24 and 48 hours.
54. In vitro assessment of the 48-hour elasticity improvement effect of Bonding Collagen T7 as a raw material.
Tests conducted by: P&K Skin Research Center Co. Ltd.
Date: 2-26 September 2024.
Sample details: Evaluation of in vitro efficacy of Bonding Collagen T7 as a raw material. Bonding Collagen T7 was tested at concentrations of 0.0001%, 0.0005%, 0.0010%, with differences observed by concentration.
55. In vitro assessment of the elasticity improvement effect of Bonding Collagen T7 compared to control materials.
Tests conducted by: P&K Skin Research Center Co. Ltd.
Date: 29 May – 4 July 2024.
Control materials: six types of collagen (T1, T3, T4, T5, T17, T21) as raw materials.
Sample details: Evaluation of in vitro efficacy of Bonding Collagen T7 as a raw material, with differences observed by concentration.
56. In vitro assessment of the elasticity improvement effect of Peptide Fiber as a raw material.
Tests conducted by: P&K Skin Research Center Co. Ltd.
Date: 2 September – 11 October 2024.
Sample details: Evaluation of in vitro efficacy of Peptide Fiber as a raw material. Peptide Fiber was tested at concentrations of 0.0001%, 0.0005%, 0.0010%, with differences observed by concentration.
57. In vitro assessment of the eye cream’s effectiveness in promoting elastin synthesis.
Tests conducted by: P&K Skin Research Center Co. Ltd.
Date: 2 September – 11 October 2024.
Test product: AHC’s “Premier Ampoule Eye Cream For Face Line Tightening” tested at concentrations of 0.0001%, 0.0005%, and 0.0010%, with differences observed by concentration.
58. In vitro assessment of the elasticity improvement effect of Bonding Collagen T7.
Tests conducted by: P&K Skin Research Center Co. Ltd.
Date: 2 September – 11 October 2024.
Sample details: Evaluation of in vitro efficacy of Bonding Collagen T7 as a raw material. Bonding Collagen T7 was tested at concentrations of 0.0001%, 0.0005%, 0.0010%, with differences observed by concentration.
59. In vitro assessment of the eye cream’s effectiveness in promoting hyaluronic acid synthesis.
Tests conducted by: P&K Skin Research Center Co. Ltd.
Date: 11-27 September 2024.
Test product: AHC’s “Premier Ampoule Eye Cream For Face Line Tightening” tested at concentrations of 0.0001%, 0.0005%, and 0.0010%, with differences observed by concentration.
Successfully passed skin safety tests:
60. Primary skin irritation test.
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 34 adults aged 20-55.
Date: 11-13 September 2024.
Treatment areas: back.
Application protocol: Patch test assessed 1 hour and 24 hours after patch removal.
Individual results may vary.
61. Primary skin irritation test for sensitive skin.
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 32 adults aged 20-55.
Date: 11-13 September 2024.
Treatment areas: back.
Application protocol: Patch test assessed 1 hour and 24 hours after patch removal.
Individual results may vary.
62. Successfully passed non-animal alternative eye irritation tests
Tests conducted by: P&K Skin Research Center Co. Ltd.
Participants: 32 adults aged 20-55.
Date: 4-6 September 2024.
Test model: in vitro human cornea-like epithelium model (MCTT HCE™).
Individual results may vary.
Directions
Take the desired amount of product and spread it evenly over the skin.
As a targeted eye treatment:
Once you have refined your skin texture using a toner, take the desired amount of product and gently tap it around the eye area until fully absorbed.
As a total facial treatment:
After completing your regular skincare routine, take a generous amount of product and spread it evenly to any wrinkled areas of your face until fully absorbed.
Usage Tips:
Precautions
- General precautions:
- Immediately consult a dermatologist if symptoms such as red blotches, swelling and/or itchiness appear while using the product, or in case such symptoms appear when the skin is exposed to direct sunlight after use.
- Do not use the product on open wounds.
- Storage and handling precautions:
- Keep out of the reach of children.
- Keep away from direct sunlight.
Other editions and formulations
Previous formulations:
Special editions:
Shop this product
International retailers:
South Korean retailers:
Make sure to check out the Discount & Coupons page to access exclusive offers for major Korean skincare retailers.
Review
n/a
